The global clinical trials market plays a crucial role in the development of new drugs and therapies, ensuring their safety and effectiveness before they reach the market. With a growing demand for innovative therapeutic solutions and increasing investments in research and development, the clinical trials market has been expanding rapidly. In 2024, the market reached a valuation of USD 52.60 billion and is projected to grow significantly in the coming decade. Technological advancements and evolving regulatory frameworks are further driving this growth, making clinical trials more efficient and accessible worldwide.
Clinical Trials Market Size
The global clinical trials market size was valued at USD 52.60 billion in 2024 and is anticipated to grow at a CAGR of 8.70% during the forecast period from 2025 to 2034. By 2034, the market is expected to achieve a value of USD 121.14 billion. This growth is primarily driven by the increasing demand for therapeutic drugs, the rising prevalence of chronic diseases, and expanding research efforts globally.
Clinical Trials Market Share
The clinical trials market is dominated by several key players who contribute significantly to market growth through extensive research capabilities and strategic collaborations. Companies such as IQVIA, PAREXEL International Corporation, and Charles River Laboratory hold substantial market shares due to their comprehensive service offerings and global reach. The increasing number of partnerships between pharmaceutical companies and clinical research organizations (CROs) also plays a vital role in consolidating market share.
Clinical Trials Market Trends
- Decentralized Clinical Trials (DCTs): The adoption of virtual and hybrid trial models is increasing, reducing the need for physical site visits.
- Incorporation of AI and Big Data: Advanced analytics and machine learning are enhancing patient recruitment and data analysis efficiency.
- Personalized Medicine: Growing focus on tailored treatments is driving demand for specialized clinical trials.
- Real-World Evidence (RWE): Utilization of real-world data is helping optimize trial design and outcomes.
Clinical Trials Market Analysis
The clinical trials market is characterized by rapid technological advancements and a robust regulatory environment. The increasing incidence of chronic diseases like cancer, diabetes, and cardiovascular conditions fuels the need for innovative drugs, leading to a surge in clinical trials. North America currently holds the largest market share due to advanced healthcare infrastructure and significant investment in R&D. Asia-Pacific is emerging as a lucrative market due to cost-effective trial processes and a growing patient population.
Get a Free Sample Report with Table of Contents
Clinical Trials Market Segmentation
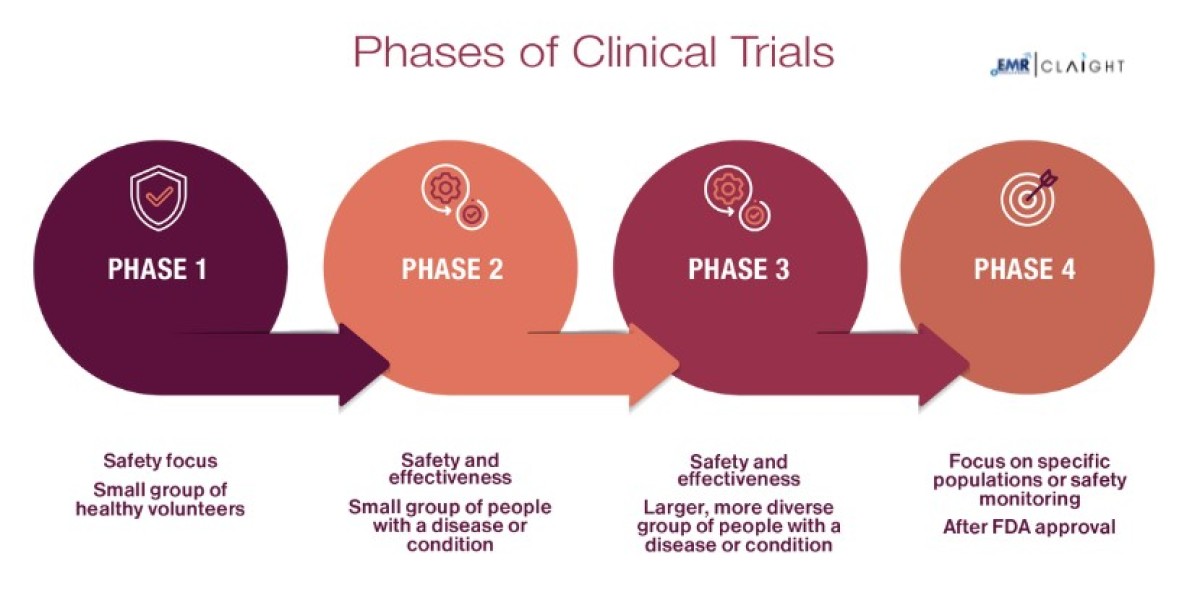
By Phase:
- Phase I
- Phase II
- Phase III
- Phase IV
By Study Design:
- Interventional Trials
- Observational Trials
- Expanded Access Trials
By Therapeutic Area:
- Oncology
- Cardiovascular
- Neurology
- Infectious Diseases
- Others
By Geography:
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East & Africa
Clinical Trials Market Growth
The clinical trials market is poised for robust growth due to rising investments in pharmaceutical R&D and increasing demand for novel therapeutic drugs. The expansion of biotechnology firms and growing collaborations between academia and industry further boost market growth. Moreover, the adoption of decentralized trials and technological integration streamline processes, reducing costs and timelines, which positively impacts market expansion.
Recent Developments and Challenges in the Clinical Trials Market
Recent Developments:
Adoption of Digital Technologies: Virtual trials and digital patient monitoring are becoming mainstream.
Regulatory Support: Faster approvals for innovative drugs are encouraging more clinical trials.
Strategic Collaborations: Increased partnerships between pharma companies and CROs enhance research capabilities.
Challenges:
High Costs: Clinical trials require substantial financial investment.
Patient Recruitment: Identifying and retaining eligible participants remains a challenge.
Regulatory Hurdles: Compliance with diverse international standards can delay trial timelines.
Key Players in the Clinical Trials Market
IQVIA
PAREXEL International Corporation
Charles River Laboratory
ICON Plc
Syneos Health Inc
Labcorp Drug Development (COVANCE)
WuXi AppTec Co., Ltd
PPD Inc.
Medpace Holdings Inc.
ACM Global Laboratories
Advanced Clinical LLC
SGS
PSI CRO AG
BioAgile Therapeutics Private Limited